Berwyn, PA – DEC. 24, 2019 – CuRAGE Therapeutics announced today that it is scheduled to deliver a company presentation at Biotech Showcase™ 2020, to be held January 13–15, during the most important week in healthcare at the Hilton San Francisco Union Square. Dr. Ihor Terleckyj, CEO, will be presenting their lead immunotherapy CR-3 antibody with disease-modifying properties for peripheral artery disease (PAD). The presentation will be held at Biotech Showcase as follows:
Date: Wednesday, January 15, 2020
Time: 10:30 am (PST)
Room: Franciscan B (Ballroom Level)
Venue: Hilton San Francisco Union Square Hotel, 333 O’Farrell Street, San Francisco, CA
“Being accepted to present at Biotech Showcase is a large milestone for CuRAGE, and we are very grateful to the selection committee for this opportunity given that thousands of companies vie for this honor”, stated Ihor Terleckyj, Ph.D, CEO, adding "This also validates the true innovation and novelty of our anti-RAGE antibody CR-3 as a drug candidate, re-affirming the critical need for real therapeutic options for patients suffering with peripheral artery disease”.
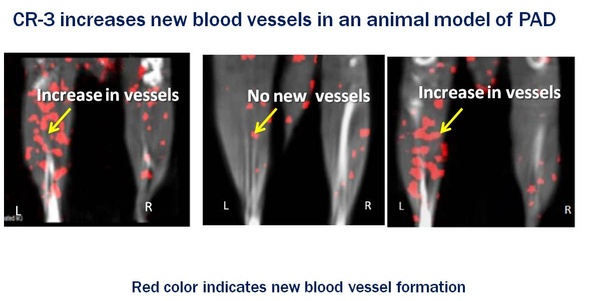
CuRAGE’s lead candidate is CR-3, a humanized monoclonal antibody developed in Dr. Lynne Johnson’s lab at Columbia University. It binds to the RAGE receptor to turn off the activity of the inflammatory pathway it controls and restores the ability to create new blood vessels. This is particularly important in the case of PAD, or peripheral artery disease, as the new vessels go around the blocked arteries which is the hallmark characteristic of this disease, to restore blood flow to the damaged tissues.
According to Dr. Johnson, “Diabetics are twice as likely to develop symptoms of PAD, especially with increased levels of glucose occurring when patients lose glucose control due to the waning of treatment with anti-diabetic agents. For each 1% increase in HbA1c, the incidence of symptomatic PAD increases by 26%. The presence of PAD in diabetics with foot ulcers is a major contributor to poor wound healing, greater morbidity, mortality and amputation".
The inflammatory activity of RAGE, or receptor for advanced glycation endproducts, leads to widespread arterial and tissue damage not only in the peripheral arteries, but also those of the heart and kidneys. In addition, RAGE causes extensive tissue damage and plays a major role in the development of atherosclerosis. "Based on all of the science, the RAGE pathway most probably is the pathological cardiovascular inflammatory process that everyone has been talking about searching for. For this reason, we feel that CR-3 should also have a positive effect on lowering the residual risk that still exists even with intensive lipid management, anti-coagulation agents and anti-diabetic drugs" said Ihor Terleckyj, CEO.
“We are delighted that CuRAGE Therapeutics is presenting their CR-3 program at Biotech Showcase this year,” said Sara Demy, CEO of Demy-Colton. “Biotech Showcase is a prime occasion for life science entrepreneurs and investors to come together to discover the potential of innovative technologies that will drive the future of drug discovery.”
ABOUT CuRAGE THERAPEUTICS
CuRAGE Therapeutics is An early-stage biotechnology company formed in October 2018 headquartered in Berwyn, PA with technology licensed from Columbia University. Our mission is to develop novel therapeutics in overlooked disease states with high unmet patient needs. We are developing a humanized anti-RAGE (receptor for advanced glycation products) monoclonal antibody CR-3 that blocks the activity of an immmuno-inflammatory pathway contributing to chronic disease development. Our lead indication is peripheral artery disease where CR-3 monoclonal antibody has demonstrated the ability to restore blood flow in both diabetic mouse and pig models of disease.
ABOUT PERIPHERAL ARTERY DISEASE
The worldwide estimate for patients with peripheral artery disease is 202 million, with 9 million in the US. The quality of life for PAD patients is poor. Due to reduced blood flow to their lower limbs, patients have reduced mobility and pain while walking or at rest. As the disease progresses, patients may require endovascular procedures or surgical bypass grafting. In the US, the annual cost of treatment and hospitalizations for PAD is over $60 billion.
Currently, there is no therapy that improves symptoms, prevents the need for revascularization procedures and delays disease progression. Statins, anticoagulants and anti-platelet agents have a large effect on reducing mortality, yet have little effect on reducing the need for endovascular procedures or surgical bypass grafting.
CuRAGE is currently seeking funding to progress CR-3 forwards into commercial drug development.
Contact:
Ihor Terleckyj, CEO
1-973-479-7110
For more information, visit us at: